Summary and recommendation
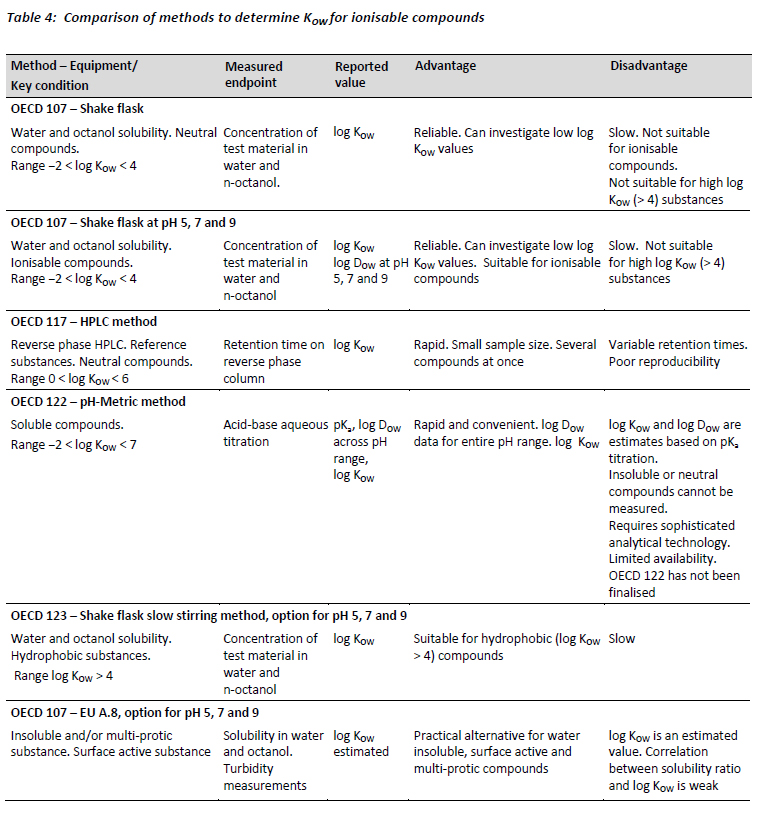
The different methods for determining KOW are compared in Table 4 and detailed in Appendix B.
The pH-metric (titration) method that is being finalised at OECD level (to be published as test guideline 122) has the potential to become the preferred method for measuring both DOW and KOW of ionisable compounds. It delivers the most comprehensive dataset of all the methods, including pKa, KOW and DOW across a wide pH range, and is applicable across a wide range of log KOW values (-2 - 7) (OECD, 2000a). However, it should be noted that KOW and DOW values are actually calculated from the measured (titrated) pKa value and apparent shift in pKa value when n-octanol is added. Nonetheless, the pH-metric procedure has been validated against the traditional standard shake-flask method and many studies using it have been reported (Slater et al, 1994; Takács-Novák and Avdeef, 1996; Chamberlain et al, 1996; Avdeef, 2001). The published literature clearly indicates that this technique is a reliable, versatile, dynamic, and an accurate method for measuring KOW (Avdeef, 2001; Kah and Brown, 2008). Other disadvantages are that, for example, the pKa must be in the measureable pH range and that the method is not applicable to neutral or insoluble compounds.
The limited availability of this method offered by contract research organisations represents an additional obstacle. The pH titration method requires rather sophisticated and expensive analytical equipment and, as such, contract research organisations are unlikely to invest in this method unless demand reaches appreciable levels. Conceivably, the EMA (2010) recommendation that log DOW should be determined as a function of pH covering an environmentally relevant pH-range, e.g. Draft Guideline OECD 122 (OECD, 2000a) will stimulate the required demand. The publication of the finalised OECD 122 (OECD, 2000a) guideline would also represent a step forward in promoting widespread acceptance of this method.
In contrast, the shake flask method (OECD 107) has traditionally been considered the ‘Gold standard'. Whilst this method is undoubtedly slow, it does deliver reproducible and accurate measurements of KOW. The method can be easily adapted to accommodate ionisable compounds by measuring DOW at pH 5, 7 and 9. It is reliable and can be used for log KOW values over a fairly wide range (-2 - 4). For hydrophobic compounds with predicted log KOW > 4, the slow stirring method OECD 123 should be used. Until the pH-metric method becomes more widely available, the shake flask method at pH 5, 7 and 9 is likely to continue to be the default choice for the measurement of KOW values for ionisable compounds.