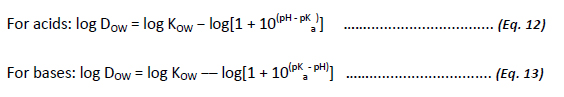Definition
The KOW is defined as the ratio of the equilibrium concentrations of a dissolved substance in a 2-phase system consisting of the largely immiscible solvents n-octanol and water, as the neutral form of the molecule (OECD, 1995a). As such, it is a measure of the hydrophobicity of the compound.
The property is moderately temperature-dependent and typically measured at 25°C.
Where [X] indicates the concentration (mass/volume) in the specific solvent.
The octanol-water distribution ratio (DOW) is a measure of KOW that accounts for the pH dependency of an ionisable organic chemical, and is a measure of the distribution of dissociated and non-dissociated species in octanol and water as a function of pH. The extent to which an ionisable compound is dissociated across environmentally relevant pH ranges may have a marked effect on properties such as water solubility. It should be noted that the neutral form of the species is generally less water soluble and is thus more hydrophobic as compared to the ionised or charged form (Avdeef, 1996).
In general, DOW can be correlated to KOW and pKa by the following relationships:
Neutral and non-ionisable organic compounds will have a DOW value that is equivalent to KOW, since DOW is a measure of the pH dependency of an ionisable organic compound. The available international standard methods are detailed and referenced in Appendix B.