Guidance on assess bio-solubility
The classes given by ICRP were based on the pulmonary clearance half-time, which is often not available. Mercer (1967) proposed a mathematical model to predict half-time of substances retained in the respiratory tract. This model is not widely applied because the required constants can only be determined from in vivo measurements. In vitro approaches cannot provide the in vivo clearance half-time, but can give an indication for the potential solubility classes. Therefore, abiotic assays have been used to assess potential bio-solubility in vivo.
The abiotic solubility assays consider that constituents of solvent have strong impact on solubility. Early simulant dissolution studies focused on the fate of aerosol particles deposited in lung extracellular fluid. The most common simulants used were based on measurements of the ionic composition of lung extracellular fluid by Gamble (Ansoborlo et al, 1999). However, particles deposited in the lower respiratory tract are most likely to be phagocytised and will be finally enclosed in secondary lysosomes or phagolysosomes. Phagolysosomes contain proteolytic enzymes, oxygen radicals, chelators, precipitators. The pH values in phagolysosmes range between 4.35 and 4.95 for baboons, dogs, guinea pigs and rabbits (Kreyling et al, 1991). Lundborg (Lundborg and Camner 1984) determined the dissolution rate of MnO2 in vitro with and without AM. They found a higher dissolution rate in the presence of AM than without. Kreyling et al (Kreyling et al, 1986; Kreyling et al, 1990) examined the parameters that influence intracellular particle dissolution using mainly Co3O4 as model substance. They considered that intracellular particle dissolution was controlled by phagolysolomal pH and chelate agents within the phagolysosomes. Based on this information, Stefaniak (Stefaniak et al, 2005) refined and characterised a potassium hydrogen phthalate (KHP) buffered solution with pH 4.55, termed phagolysosomal simulant fluid (PSF), for use in a static dissolution technique.
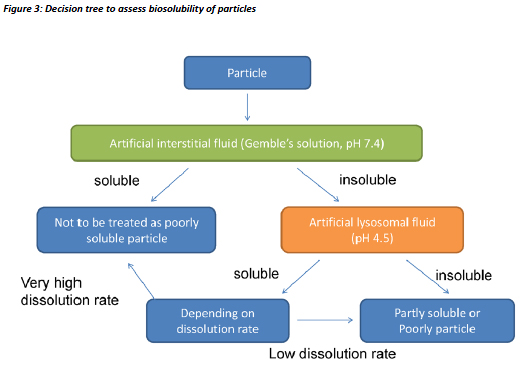
Based on these published data, we suggest determining the solubility of particles in Gamble´s solutions and/or PSF. The following decision tree (Figure 3) illustrates how to assess the biosolubility of a test substance based on the results of the abiotic solubility assays.
However, abiotic solubility assays may underestimate in vivo biosolubility for the following reasons:
1) Solubility is defined as the concentration in the liquid at equilibrium, when dissolution rate equals precipitation rate. In vivo within the respiratory tract, renewal of the lining fluid is continuous, and therefore particle dissolution does not reach equilibrium.
2) Dissolution is a dynamic process in which constituent molecules of the dissolving solid migrate from the surface to the bulk solution through a dissolution layer (Borm et al, 2006). Dissolution rate increases, when particles become smaller during the course of dissolution.
Thus, when using abiotic solubility data to assess in vivo solubility, these uncertainties have to be considered.